Media & Events (2)
Latest News
UPM Pharmaceuticals Board of Directors CEO Interview | Read This Interview With UPM Pharmaceuticals Board of Directors Ceo, Jim Gregory
Facilitating Implementation and Customer Service with Offline Serialization
UPM’s VP of Quality Control Daniel Dixon | Learn About UPM’s Newest VP of Quality Control, Daniel Dixon
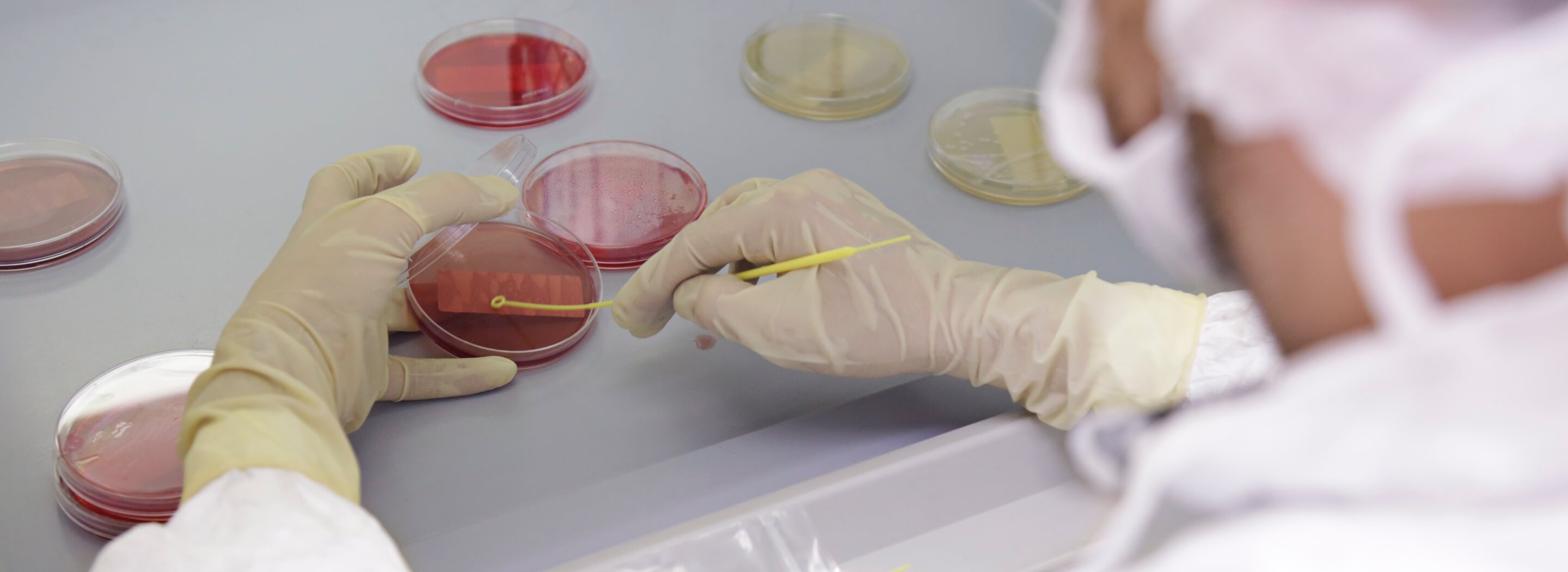
UPM’s CDMO Drug Development Services | Learn About UPM’s CDMO Formulation Development Services & Process
UPM’s New COO Chris Curtin | Learn About How UPM Appointed Chris Curtin to UPM Management Team As COO
Contractors: Challenge of Complex Molecules
UPM Pharmaceutical Sterilization of First Product | Read About the First Sterilization of Pharmaceutical Products
Anticipating What’s Around the Bend
Moving Quality to the Forefront Brings Measureable Results
100 Years of UPM at its Bristol Manufacturing Site | Read About UPM Celebrating 100 Years of its Bristol Manufacturing Site